Mobil1 has reportedly met the SN PLus/d1G2 standards for years using the low calcium, high magnesium add packs now adopted by almost all of its competitors. As Mobil1seems to be performing just fine, I’d say the new standards have no downside. And even if you aren’t concerned about LSPI, d1G2 enhances timing chain wear protection.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Known downsides to LSPI-oriented formulations?
- Thread starter d00df00d
- Start date
- Status
- Not open for further replies.
I think the answer to this is that they don't entirely know why. Most of the explanations I have heard are just that calcium seems to increase the likelihood of events, but the exact reason isn't known.
wemay
Site Donor 2023
Originally Posted By: Shannow
Originally Posted By: CR94
Originally Posted By: dparm
Originally Posted By: CR94
The biggest downside might be increased cost, for customers who didn't need to worry about LSPI in the first place.
Why would consumers not need to worry about LSPI? Are you saying that it's not something the average person needs to think about, or that it's not even happening?
No, I'm saying LSPI is something consumers who won't be using the oil in engines vulnerable to LSPI don't need to worry about. They'll have to help pay for the changes, though.
Yes...
and people whose vehicle manufacturer doesn't have a habit of oil burning have to put up with lower catalyst poisoning additives because of those that state 1qt/1,000 miles is "acceptable".
The expeniseve engine tests are carried out on engines that have a "problem" that reliably responds to certain factors in an engine oil...you pay for that testing too, even 'though yours might not have THAT particular Achilles Heel.
Best post in this thread.
Originally Posted By: CR94
Originally Posted By: dparm
Originally Posted By: CR94
The biggest downside might be increased cost, for customers who didn't need to worry about LSPI in the first place.
Why would consumers not need to worry about LSPI? Are you saying that it's not something the average person needs to think about, or that it's not even happening?
No, I'm saying LSPI is something consumers who won't be using the oil in engines vulnerable to LSPI don't need to worry about. They'll have to help pay for the changes, though.
Yes...
and people whose vehicle manufacturer doesn't have a habit of oil burning have to put up with lower catalyst poisoning additives because of those that state 1qt/1,000 miles is "acceptable".
The expeniseve engine tests are carried out on engines that have a "problem" that reliably responds to certain factors in an engine oil...you pay for that testing too, even 'though yours might not have THAT particular Achilles Heel.
Best post in this thread.
Originally Posted By: JAG
You don’t want good TBN retention. You want the bases to neutralize as much of the acids as possible and as quickly as possible. By laws of nature, this reduces the amount of available base...TBN goes down. You do want enough TBN but not due it to “retaining” it; you want it due to it starting at a high value for your OCI. You want TAN to increase as little as possible.
I don’t like Mg detergents because of the following paper: http://www.oil-lab.com/downloads/TBN-1.pdf
I was the one who posted that paper (link to the post).
I also initially thought that the Mg detergents should be avoided and Ca detergents are the best. However, later, I realized that the ideal detergent is when you have both Ca and Mg. In fact, this was the conclusion of that old paper anyway.
This is because a Ca-only detergent neutralizes all types of acids and depletes very quickly. Once it depletes, your engine is in jeopardy with very high wear numbers due to acid corrosion.
Yes, a Mg-only detergent fails to neutralize all types of acids, and this is bad for your engine as well.
However, if you have both Ca and Mg, you get best of both worlds. You still neutralize all types of acids, which wouldn't be the case for a Mg-only detergent, and your detergent works longer (good TBN retention), which wouldn't be the case for a Ca-only detergent.
Therefore, a detergent that contains both Ca and Mg in similar amounts is absolutely the best detergent you can get. Of course, any detergent needs to go through many engine tests to make sure it's compatible with the antiwear additives.
Answering the OP, LSPI-protection oils that contain both Ca and Mg have the best detergent system you can get. They not only protect against LSPI but offer great protection against corrosion and wear since Ca and Mg work in synergy to make up for each other's weaknesses as I explained above.
So, no, there are no downsides to LSPI-orientated formulations!
You don’t want good TBN retention. You want the bases to neutralize as much of the acids as possible and as quickly as possible. By laws of nature, this reduces the amount of available base...TBN goes down. You do want enough TBN but not due it to “retaining” it; you want it due to it starting at a high value for your OCI. You want TAN to increase as little as possible.
I don’t like Mg detergents because of the following paper: http://www.oil-lab.com/downloads/TBN-1.pdf
I was the one who posted that paper (link to the post).
I also initially thought that the Mg detergents should be avoided and Ca detergents are the best. However, later, I realized that the ideal detergent is when you have both Ca and Mg. In fact, this was the conclusion of that old paper anyway.
This is because a Ca-only detergent neutralizes all types of acids and depletes very quickly. Once it depletes, your engine is in jeopardy with very high wear numbers due to acid corrosion.
Yes, a Mg-only detergent fails to neutralize all types of acids, and this is bad for your engine as well.
However, if you have both Ca and Mg, you get best of both worlds. You still neutralize all types of acids, which wouldn't be the case for a Mg-only detergent, and your detergent works longer (good TBN retention), which wouldn't be the case for a Ca-only detergent.
Therefore, a detergent that contains both Ca and Mg in similar amounts is absolutely the best detergent you can get. Of course, any detergent needs to go through many engine tests to make sure it's compatible with the antiwear additives.
Answering the OP, LSPI-protection oils that contain both Ca and Mg have the best detergent system you can get. They not only protect against LSPI but offer great protection against corrosion and wear since Ca and Mg work in synergy to make up for each other's weaknesses as I explained above.
So, no, there are no downsides to LSPI-orientated formulations!
In the paper the Ca/Mg ratios were 6 and 3, which is larger than all of the d1G2 oils I recall seeing. Mobil 1’s ratio is around 1.5 to 2. The one UOA I looked at of Castrol synthetic had a ratio of around 0.8. Those ratios are significantly lower than the ratios tested, except for all Mg.
In terms of TAN and lead corrosion, performance (not all present in every test) from highest to lowest was: all Calcium, 6:1 ratio Ca:Mg, 3:1 ratio Ca:Mg, all Mg. Lead corrosion is what is most important and even oils having same TAN can cause different amount of corrosiveness. Copper also matters but they did not test for it. TBN has a moderate to high degree of deceptiveness/irrelevance. I’d always choose TAN test over a TBN test. That, in combination with lead and copper values tells a lot. The potential interference is rubbing wear of lead and/or copper bearings. If a person gets a UOA on the same oil at different mileage’s across the way, he/she can look at the curve of lead and copper vs. mileage, and if it shows a significant and increasing rate (PPM/mile) over multiple sucesssive UOAs at OCIs long enough really tax the oil, it would likely indicate acid-induced corrosion has started to occur. To conclude that, it must be determined that excessive dirt ingestion is not the cause of increased rubbing wear.
In terms of TAN and lead corrosion, performance (not all present in every test) from highest to lowest was: all Calcium, 6:1 ratio Ca:Mg, 3:1 ratio Ca:Mg, all Mg. Lead corrosion is what is most important and even oils having same TAN can cause different amount of corrosiveness. Copper also matters but they did not test for it. TBN has a moderate to high degree of deceptiveness/irrelevance. I’d always choose TAN test over a TBN test. That, in combination with lead and copper values tells a lot. The potential interference is rubbing wear of lead and/or copper bearings. If a person gets a UOA on the same oil at different mileage’s across the way, he/she can look at the curve of lead and copper vs. mileage, and if it shows a significant and increasing rate (PPM/mile) over multiple sucesssive UOAs at OCIs long enough really tax the oil, it would likely indicate acid-induced corrosion has started to occur. To conclude that, it must be determined that excessive dirt ingestion is not the cause of increased rubbing wear.
Originally Posted By: JAG
In the paper the Ca/Mg ratios were 6 and 3, which is larger than all of the d1G2 oils I recall seeing. Mobil 1’s ratio is around 1.5 to 2. The one UOA I looked at of Castrol synthetic had a ratio of around 0.8. Those ratios are significantly lower than the ratios tested, except for all Mg.
In terms of TAN and lead corrosion, performance (not all present in every test) from highest to lowest was: all Calcium, 6:1 ratio Ca:Mg, 3:1 ratio Ca:Mg, all Mg. Lead corrosion is what is most important and even oils having same TAN can cause different amount of corrosiveness. Copper also matters but they did not test for it. TBN has a moderate to high degree of deceptiveness/irrelevance. I’d always choose TAN test over a TBN test. That, in combination with lead and copper values tells a lot. The potential interference is rubbing wear of lead and/or copper bearings. If a person gets a UOA on the same oil at different mileage’s across the way, he/she can look at the curve of lead and copper vs. mileage, and if it shows a significant and increasing rate (PPM/mile) over multiple sucesssive UOAs at OCIs long enough really tax the oil, it would likely indicate acid-induced corrosion has started to occur. To conclude that, it must be determined that excessive dirt ingestion is not the cause of increased rubbing wear.
Mobil 1 Ca/Mg ratio is 4/3, not 3/4. You got it mixed.
https://www.bobistheoilguy.com/forums/ubbthreads.php/topics/4389559/Mobil_1_0W-20_Annual_Protectio
https://www.bobistheoilguy.com/forums/ubbthreads.php/topics/2243763/VOA_with_TBN_and_TAN_for_Mobil
That paper's study is extremely flawed. Their mostly Ca oil is high-TBN versus all-Mg oil is low-TBN. If you look at the results, the main indicator of lead corrosion is remaining TBN. Ca is working better only because it has a much higher starting TBN. When TBN goes below a certain point, lead corrosion increases dramatically. Once the TBN is depleted, the oil no longer protects regardless of Ca or Mg and the engine starts experiencing great wear rates. That's why TBN retention is crucial. The only way you can have TBN retention is either a high-SAPS oil (ACEA A3/B4) or a Mg-containing detergent. Since the former isn't allowed for most modern engines, you are stuck with the latter.
Also, the very final sentence in the paper is "The best formulating approach utilizes higher TBN and a mixed calcium/magnesium." (What's up with all the typos in this paper?)
When I was going over the paper again, one thing struck me though: Mg-containing detergents are greatly increasing the oil oxidation. I don't understand this but if this is true, it would certainly be a significant downside of Mg. This also reminds me Delvac, which is Ca/Mg-based, failing the Volvo T-13 oxidation test according to Rotella, which is Ca-only-based. Could it be because of Mg? It just arises some suspicion after reading this.
So, apart from the caveat regarding oil oxidation, a well-balanced Ca/Mg detergent is clearly superior to an all-Ca detergent in terms of neutralizing acids throughout an extended OCI. The oil-oxidation concerns of Mg need further thought and investigation.
In the paper the Ca/Mg ratios were 6 and 3, which is larger than all of the d1G2 oils I recall seeing. Mobil 1’s ratio is around 1.5 to 2. The one UOA I looked at of Castrol synthetic had a ratio of around 0.8. Those ratios are significantly lower than the ratios tested, except for all Mg.
In terms of TAN and lead corrosion, performance (not all present in every test) from highest to lowest was: all Calcium, 6:1 ratio Ca:Mg, 3:1 ratio Ca:Mg, all Mg. Lead corrosion is what is most important and even oils having same TAN can cause different amount of corrosiveness. Copper also matters but they did not test for it. TBN has a moderate to high degree of deceptiveness/irrelevance. I’d always choose TAN test over a TBN test. That, in combination with lead and copper values tells a lot. The potential interference is rubbing wear of lead and/or copper bearings. If a person gets a UOA on the same oil at different mileage’s across the way, he/she can look at the curve of lead and copper vs. mileage, and if it shows a significant and increasing rate (PPM/mile) over multiple sucesssive UOAs at OCIs long enough really tax the oil, it would likely indicate acid-induced corrosion has started to occur. To conclude that, it must be determined that excessive dirt ingestion is not the cause of increased rubbing wear.
Mobil 1 Ca/Mg ratio is 4/3, not 3/4. You got it mixed.
https://www.bobistheoilguy.com/forums/ubbthreads.php/topics/4389559/Mobil_1_0W-20_Annual_Protectio
https://www.bobistheoilguy.com/forums/ubbthreads.php/topics/2243763/VOA_with_TBN_and_TAN_for_Mobil
That paper's study is extremely flawed. Their mostly Ca oil is high-TBN versus all-Mg oil is low-TBN. If you look at the results, the main indicator of lead corrosion is remaining TBN. Ca is working better only because it has a much higher starting TBN. When TBN goes below a certain point, lead corrosion increases dramatically. Once the TBN is depleted, the oil no longer protects regardless of Ca or Mg and the engine starts experiencing great wear rates. That's why TBN retention is crucial. The only way you can have TBN retention is either a high-SAPS oil (ACEA A3/B4) or a Mg-containing detergent. Since the former isn't allowed for most modern engines, you are stuck with the latter.
Also, the very final sentence in the paper is "The best formulating approach utilizes higher TBN and a mixed calcium/magnesium." (What's up with all the typos in this paper?)
When I was going over the paper again, one thing struck me though: Mg-containing detergents are greatly increasing the oil oxidation. I don't understand this but if this is true, it would certainly be a significant downside of Mg. This also reminds me Delvac, which is Ca/Mg-based, failing the Volvo T-13 oxidation test according to Rotella, which is Ca-only-based. Could it be because of Mg? It just arises some suspicion after reading this.
So, apart from the caveat regarding oil oxidation, a well-balanced Ca/Mg detergent is clearly superior to an all-Ca detergent in terms of neutralizing acids throughout an extended OCI. The oil-oxidation concerns of Mg need further thought and investigation.
How much does ZDDP play in here?
I have a distant memory of reading that it can lower the risk in high Ca oils.
I really like the "new" Rotella T6 5w40 in my VW FSI 2.0T engine and it has high Ca and a good dose of ZDDP, low Mg.
ZDDP should be beneficial for the silly high pressure fuel pumps Cam Follower..
https://www.bobistheoilguy.com/forums/ub...SM_#Post4490450
I have a distant memory of reading that it can lower the risk in high Ca oils.
I really like the "new" Rotella T6 5w40 in my VW FSI 2.0T engine and it has high Ca and a good dose of ZDDP, low Mg.
ZDDP should be beneficial for the silly high pressure fuel pumps Cam Follower..
https://www.bobistheoilguy.com/forums/ub...SM_#Post4490450
Originally Posted By: Gokhan
The only way you can have TBN retention is either a high-SAPS oil (ACEA A3/B4) or a Mg-containing detergent. Since the former isn't allowed for most modern engines, you are stuck with the latter.
Replace “modern” with “CAFE” and I could agree.
Also you forgot about low sulfur fuel and blow by.
The only way you can have TBN retention is either a high-SAPS oil (ACEA A3/B4) or a Mg-containing detergent. Since the former isn't allowed for most modern engines, you are stuck with the latter.
Replace “modern” with “CAFE” and I could agree.
Also you forgot about low sulfur fuel and blow by.
Originally Posted By: nap
Originally Posted By: Gokhan
The only way you can have TBN retention is either a high-SAPS oil (ACEA A3/B4) or a Mg-containing detergent. Since the former isn't allowed for most modern engines, you are stuck with the latter.
Replace “modern” with “CAFE” and I could agree.
Also you forgot about low sulfur fuel and blow by.
This is not relevant to the OP's question regarding LSPI-oriented oils.
CAFE? No. Low-SAPS oils have originated in Europe, not America. They are because of the Euro emissions standards (currently Euro 6), in order to protect the emissions components, particularly the particulate filters. North American oils are mid-SAPS, not low-SAPS. Why would you want an oil that could harm your expensive emissions components? As I keep pointing out, motor oil is always about compromise. There is no such thing as increasing the SAPS indefinitely being good for your engine.
Low-sulfur fuel means you have less sulfuric acid produced during combustion, which reduces the need for high initial TBN. Alternatively, you can do longer OCI's with the same oil with low-sulfur fuel, such as in California and Europe, meaning your TBN stays high longer with low-sulfur fuel. It's definitely a good thing.
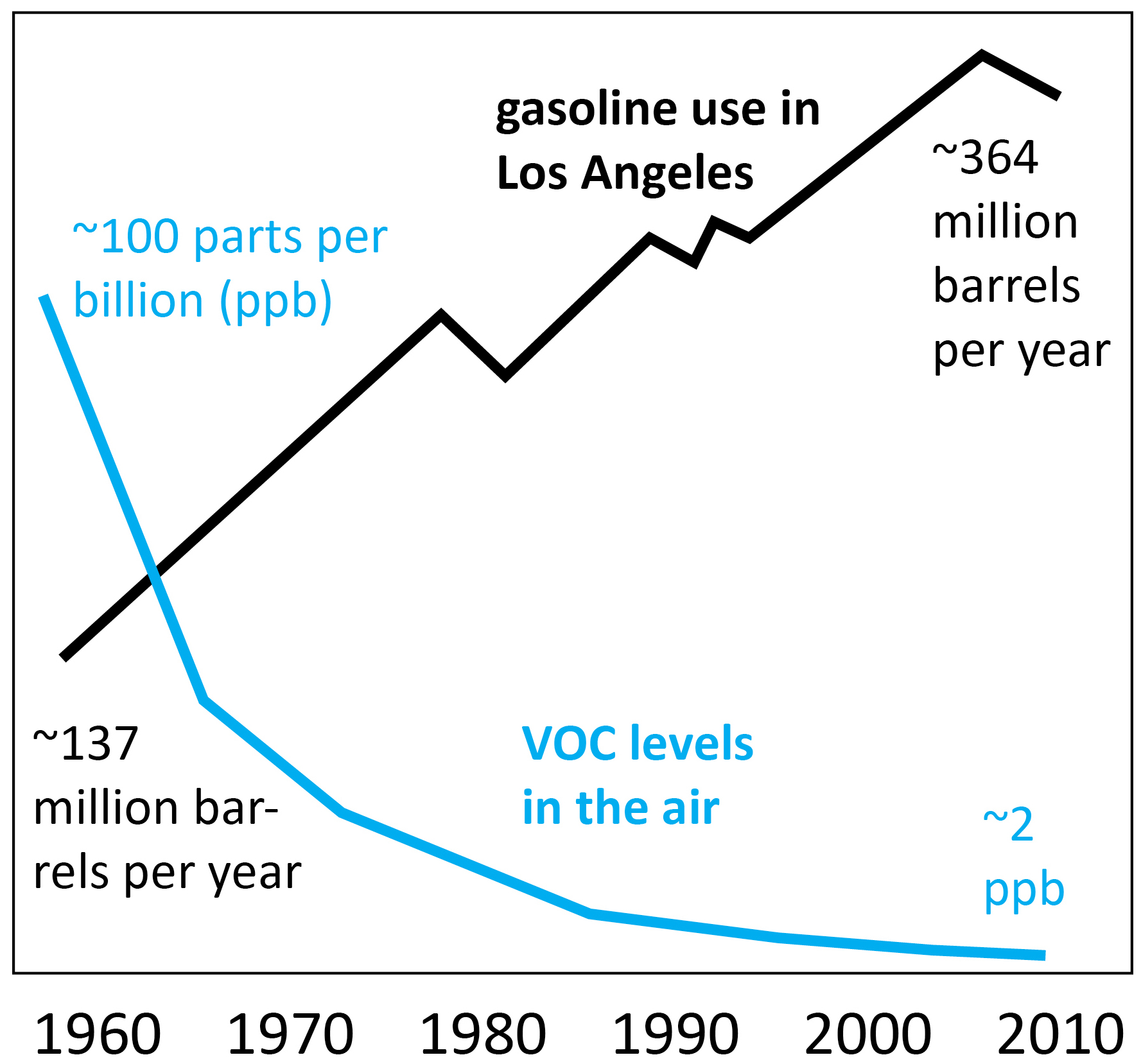
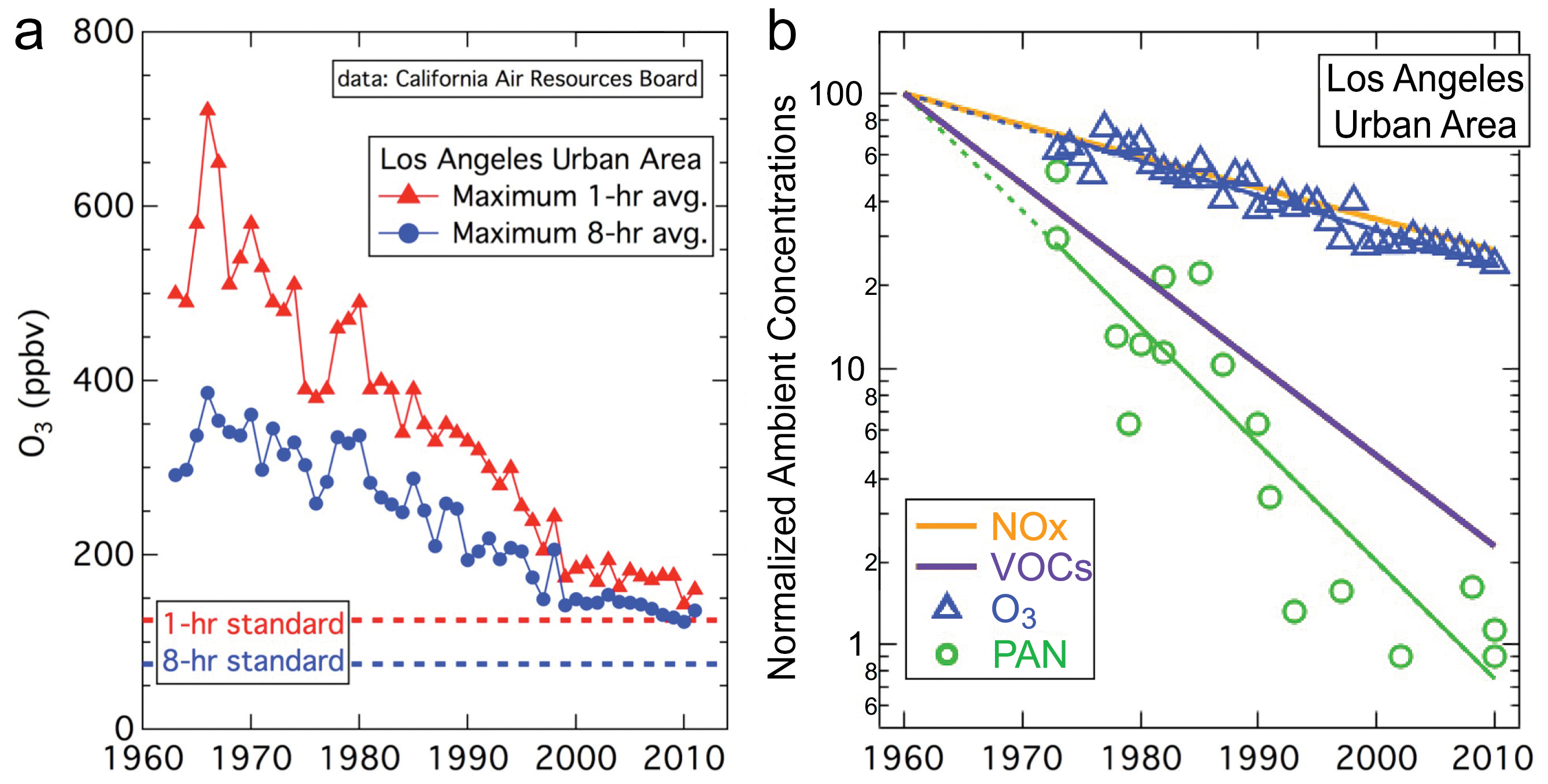
Do you want to release the PCV gases into the air? PCV has been around for many decades. Do you want to go back to the 1960s and 1970s? Have you lived in Los Angeles in the past with the thick red smog clouds, resembling the Martian atmosphere? Most people live in the cities these days, not in the open country where the emissions are not a concern.

Originally Posted By: Gokhan
The only way you can have TBN retention is either a high-SAPS oil (ACEA A3/B4) or a Mg-containing detergent. Since the former isn't allowed for most modern engines, you are stuck with the latter.
Replace “modern” with “CAFE” and I could agree.
Also you forgot about low sulfur fuel and blow by.
This is not relevant to the OP's question regarding LSPI-oriented oils.
CAFE? No. Low-SAPS oils have originated in Europe, not America. They are because of the Euro emissions standards (currently Euro 6), in order to protect the emissions components, particularly the particulate filters. North American oils are mid-SAPS, not low-SAPS. Why would you want an oil that could harm your expensive emissions components? As I keep pointing out, motor oil is always about compromise. There is no such thing as increasing the SAPS indefinitely being good for your engine.
Low-sulfur fuel means you have less sulfuric acid produced during combustion, which reduces the need for high initial TBN. Alternatively, you can do longer OCI's with the same oil with low-sulfur fuel, such as in California and Europe, meaning your TBN stays high longer with low-sulfur fuel. It's definitely a good thing.
Do you want to release the PCV gases into the air? PCV has been around for many decades. Do you want to go back to the 1960s and 1970s? Have you lived in Los Angeles in the past with the thick red smog clouds, resembling the Martian atmosphere? Most people live in the cities these days, not in the open country where the emissions are not a concern.



Calsium protects to heat too. And is used in other products for Thermal resistance.
Originally Posted By: nap
Also used in Tums tablets lol I wonder if you could use them to treat your oil

Calcium carbonate in your motor oil?
Also used in Tums tablets lol I wonder if you could use them to treat your oil

Calcium carbonate in your motor oil?
Darn it, I wasn’t originally wrong about M1’s CA/Mg ratio. Dagnubit Gokhan!

Anyhow, metallic detergents are made oil soluble by them having a hydrocarbon tail.

Anyhow, metallic detergents are made oil soluble by them having a hydrocarbon tail.
I haven't looked up the link in quite some time, but wemay posted some time ago about a study that linked an increase of magnesium based detergents along with a reduction of those based on calcium to an interference with the synergistic behavior of MoS2 and ZDDP in forming a protective "glass" on metal surfaces in engines, leading to higher wear results.
Luckily, the paper also reported that boron-based additives (I believe for AW/EP) helped to restore the proper synergy of MoS2 and ZDDP even in the presence of the magnesium detergents. I have to admit that I was a little concerned after seeing this paper since I have been using low calcium, high magnesium formulations for some time due to LSPI concerns about my DIT engine, but I suspect that the d1G2 formulations we can buy now should have this possible effect under control.
Luckily, the paper also reported that boron-based additives (I believe for AW/EP) helped to restore the proper synergy of MoS2 and ZDDP even in the presence of the magnesium detergents. I have to admit that I was a little concerned after seeing this paper since I have been using low calcium, high magnesium formulations for some time due to LSPI concerns about my DIT engine, but I suspect that the d1G2 formulations we can buy now should have this possible effect under control.
- Status
- Not open for further replies.
Similar threads
- Locked
- Sticky
- Replies
- 22
- Views
- 13K
- Replies
- 7
- Views
- 2K
- Locked
- Replies
- 17
- Views
- 1K
- Locked
- Replies
- 4
- Views
- 17K


