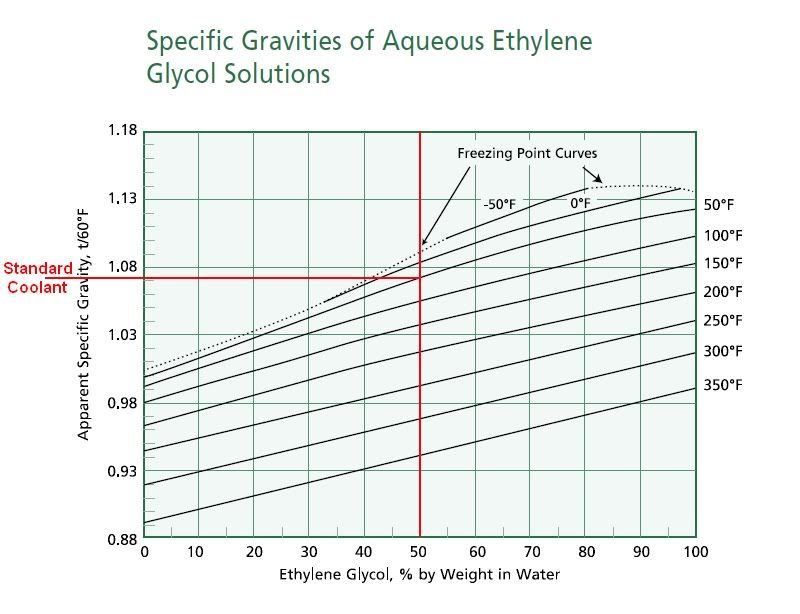
Anyone care to guess what pure dexcool will show for freeze point?
I read awhile back that straight antifreeze froze at zero degrees Fahrenheit. So, would my Prestone gauge/monitor be able to accuratey gauge it?
Would temp do you think the gauge will display as the freezing point/boilover for concentrate?
I think it will be off the charts sinc I think it is thicker that plain water and will thus give false readings....kind of like tricking the censor-bot.
I read awhile back that straight antifreeze froze at zero degrees Fahrenheit. So, would my Prestone gauge/monitor be able to accuratey gauge it?
Would temp do you think the gauge will display as the freezing point/boilover for concentrate?
I think it will be off the charts sinc I think it is thicker that plain water and will thus give false readings....kind of like tricking the censor-bot.