Originally Posted By: UrS4
Ok guys. first I have to point out that I'm not trying to offend anyone at all. If someone find this post offending then please excuse me. Also English is my 2nd language so please bear with me.
Why is so many so focussed on the "two flame fronts colliding" theory regarding detonation..?!! I guess it's a theory posted in some old books about SI engines.
By searching the web I can't really find any valid proof to back up this theory (if you know about some decent evidence on this theory I would really like to read it) - what I mostly get is mechanics, backyard mechanics and car enthusiasts arguing back and forth - the thing they typically have in common is that they lack basic understanding of thermodynamics.
Try to look at it this way..
Let's say we have an engine operating at high load (high cylinder filling),
1. As the air fuel mix is being compressed in the cylinder the mixture will then heat up but not so much that it will self ignite
2. The spark plug fires at the time it is supposed to, say 20 deg. before tdc, and the air fuel mix is lit
3. The flame progresses from the spark plug like a growing hemisphere.
From the moment the fire is lit till several deg. after tdc the temperature in the cylinder rises remarkably and therefore the pressure will also rise. Meanwhile the piston will also contribute a little to the pressure and temp gain on its last way to tdc.
4. Air fuel mix is lit but flame front has not reached the outer edges of the combustion chamber and the piston has passed tdc, okay.
4.1 Now.. Imagine that the air-fuel temperature near the piston edge and cylinder wall gets so hot that it can't resist auto ignition any longer and then makes virtually all the unburnt air fuel mix detonate at the same time which will make a super rapid increase in pressure that potentiality may damage the engine. I will kinda claim that it happens so fast that the that pressure develop and collapse so fast that it will end up being a sonic pressure wave bouncing back and forth in the chamber till the pressures has equalized.
Lets say we have some glowing deposits in the combustion chamber that starts the burn before the spark plug fires..(That's what I call pre-ignition) Then the temperature rise rapidly much earlier and create much larger pocket of air and fuel that will detonate and create even higher pressures that may cause catastrophic engine damage
I don't require anyone to agree with my view on pinging/detonation in SI engines. But I hope someone will find it interesting and maybe dig deeper into the processes and phenomenons happening in SI engines.
Maybe someone got a deeper insight of what exactly goes on during detonation in SI engines. If so I will encourage you to share it so that we learn.
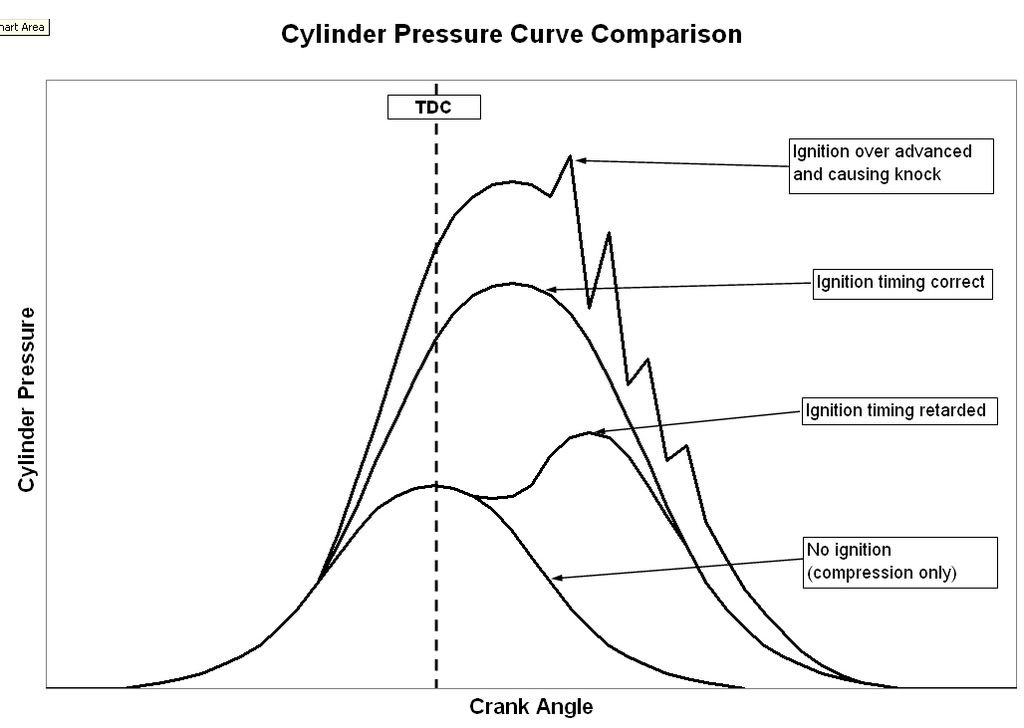
Good insight! Good graph! Your English is good! Don't worry, nobody is going to execute you!

I think that graph might need to be adjusted just a little bit. How is detonation going to occur if the cylinder pressure is decreasing as indicated in the graph? I suppose heat could be increasing due to burning gasoline, but pressure is going down due to piston movement? That is the only thing I can think of.
Perhaps the two flame fronts comment is not accurate. Detonation is just a shock due to all the fuel combusting all at once, creating a force so great that it will crack pistons and rings.