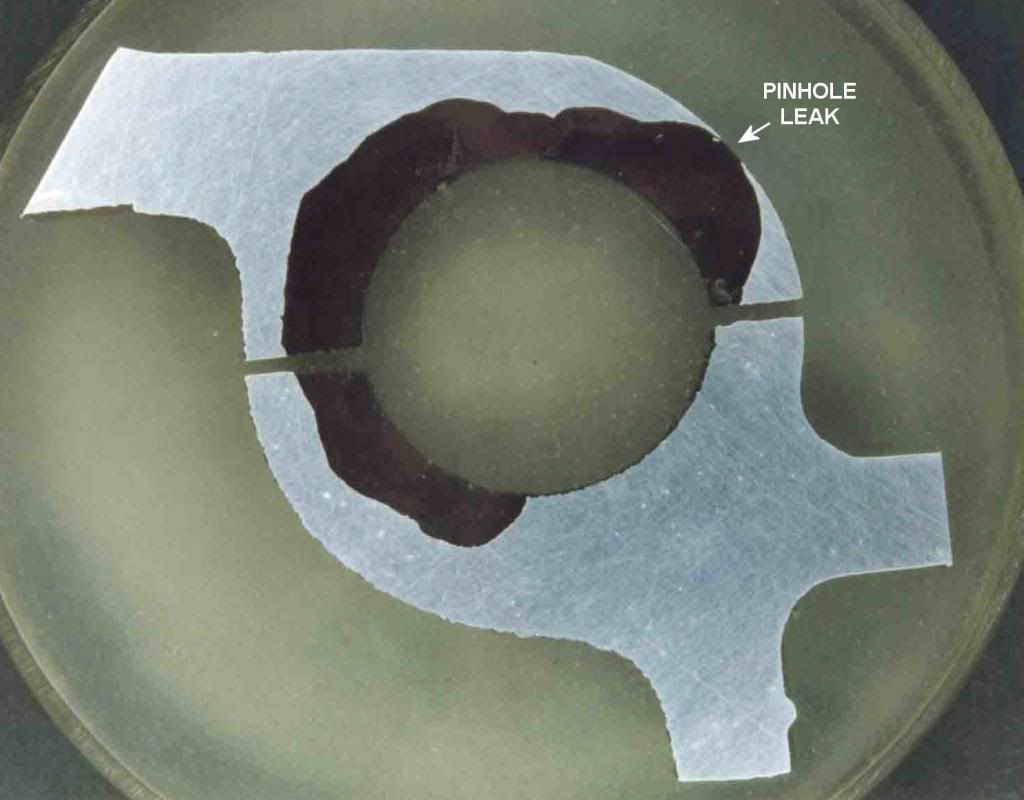
' morning all...I just had a Homer Simpson :doh: moment...been working in power stations, steam cycle for a quarter century, and today read something so simple and correct....
Quote:
Nucleate Boiling: by allowing the boundary layer of the coolant jacket to operate at its boiling temperature, the heat transfer coefficient is enhanced, making the system more efficient at removing heat and allowing lower pump work through a lower flow requirement. Figure 2 shows the heat flux as a function of wall surface temperature. As the fluid near the surface begins to boil the heat flux is improved as illustrated by the change in gradient in the curves. In this condition, small bubbles of vapour form at the convective surface of the fluid. As the vapour forms it leaves the boundary layer and moves up into the bulk fluid. The bulk fluid being at a lower temperature, the vapour bubbles quickly condensate back into the fluid. It is important that this occurs and that a level of saturated boiling does not occur: in this case the bulk fluid temperature would be at boiling temperature and large pockets of vapour would form which could rapidly lead to coolant spill and catastrophic failure [10]. Very few engines are designed for nucleate boiling and it is often used as a safety margin. Lee and Cholewczynski [23] estimated that under severe driving conditions 60% of heat absorbing surfaces in the coolant jacket experienced nucleate boiling. This was estimated by looking at changes in temperatures at different coolant pressures. It is important to note that if nucleate boiling were to occur, this would need to be accounted for in CFD models to avoid inaccuracies [24]. In addition, it would be anticipated that components that did experience nucleate boiling would be relatively insensitive to bulk coolant temperature.
Paper on fuel efficiency in OTR transport.
When it comes to shifting heat, allowing nucleate boiling moves 20 times as much thermal energy as a 5C change in coolant temperature...as stated, a blanketting of steam (departure from nucleate boiling...boiler tubes last literally second in that case) is bad.
Quote:
Nucleate Boiling: by allowing the boundary layer of the coolant jacket to operate at its boiling temperature, the heat transfer coefficient is enhanced, making the system more efficient at removing heat and allowing lower pump work through a lower flow requirement. Figure 2 shows the heat flux as a function of wall surface temperature. As the fluid near the surface begins to boil the heat flux is improved as illustrated by the change in gradient in the curves. In this condition, small bubbles of vapour form at the convective surface of the fluid. As the vapour forms it leaves the boundary layer and moves up into the bulk fluid. The bulk fluid being at a lower temperature, the vapour bubbles quickly condensate back into the fluid. It is important that this occurs and that a level of saturated boiling does not occur: in this case the bulk fluid temperature would be at boiling temperature and large pockets of vapour would form which could rapidly lead to coolant spill and catastrophic failure [10]. Very few engines are designed for nucleate boiling and it is often used as a safety margin. Lee and Cholewczynski [23] estimated that under severe driving conditions 60% of heat absorbing surfaces in the coolant jacket experienced nucleate boiling. This was estimated by looking at changes in temperatures at different coolant pressures. It is important to note that if nucleate boiling were to occur, this would need to be accounted for in CFD models to avoid inaccuracies [24]. In addition, it would be anticipated that components that did experience nucleate boiling would be relatively insensitive to bulk coolant temperature.
Paper on fuel efficiency in OTR transport.
When it comes to shifting heat, allowing nucleate boiling moves 20 times as much thermal energy as a 5C change in coolant temperature...as stated, a blanketting of steam (departure from nucleate boiling...boiler tubes last literally second in that case) is bad.