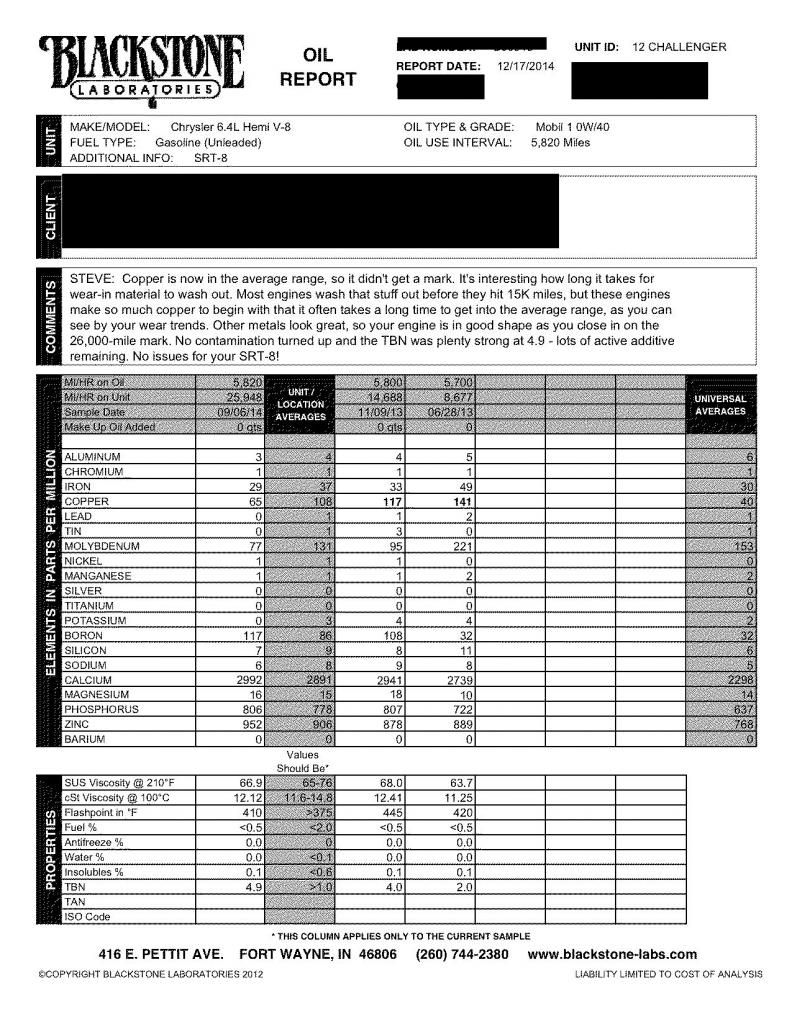
Originally Posted By: CentAmDL650
Originally Posted By: KGMtech
Interesting about the Cu levels. Yours is going the right way. If the Cu comes from bearings, you would expect to have much higher levels of tin and lead in your history if the bearings were wearing into their happy spots.
Cu is a backing metal for bearings, it is covered with tin / lead overlay, so bearing wear would exhibit high tin & lead before the Cu got onto the UOA. Unless I have my head up somewhere dark?
Many oil coolers are copper construction, and after fabrication in mass produced engines, they are likely not ultrasonic cleaned before being placed in service. So Cu particles break free and get into the oil.
Aluminum (and occasionally silver) are also used as bearing-backing materials.
In the case of coolers, the ones that have Cu exposed surfaces, the initial installation will not have loose particles ~ this would be checked ~ trust me on this, I've worked in auto industry for over 20 years. Besides, the oil filter will catch particles, so these will not be part of the UOA.
Having said that, the exposed copper surfaces do react with the oil on the first fill and then form an oxide that stops further exposure.
I think there might be another source for the Cu, perhaps it is assembly lube /anti seize that is flushing out?